Condensation Polymerisation (GCSE Chemistry): What you need to know
Condensation polymerisation is a fascinating process that drives the creation of everyday materials like plastics we rely on daily. Have you ever wondered how they’re made?
In simple terms, condensation polymerisation is a chemical reaction where small molecules, called monomers, join together to form long chains called polymers. What’s unique about this process is that every time two monomers bond, a small byproduct, like water, is released.
This process is not just important in chemistry but is also a key topic in GCSE Chemistry. Understanding condensation polymerisation helps students grasp how materials like plastics, nylon, and even proteins are made. It also answers questions like, “How does polymerisation work?” and “Where do plastics come from?”
In this blog, we’ll explain how condensation polymerisation works and explore examples like polyester and nylon. We’ll also discuss its importance in biology and plastic production. By the end, you’ll understand this essential process and its role in chemistry and daily life. Let’s dive in!
What is Condensation Polymerisation?
At its core, condensation polymerisation is a chemical reaction where small molecules, called monomers, join together to form long chains known as polymers. What sets it apart is that every time two monomers bond, a small molecule, like water or methanol, is released as a byproduct.
This is very different from addition polymerisation, where monomers join without releasing any byproducts. In condensation polymerisation, the monomers involved must have at least two functional groups, such as carboxylic acids, alcohols, or amines, that react with each other to form the polymer.
For example, in the production of polyesters, a dicarboxylic acid reacts with a diol (a type of alcohol), forming a long polymer chain while releasing water molecules. Similarly, polyamides like nylon are made by reacting a diamine with a dicarboxylic acid, again releasing water during the reaction.
Here’s the simple mechanism:
- Step 1: Monomers with two functional groups come together.
- Step 2: The functional groups react, forming bonds and releasing small molecules like water.
- Step 3: This process repeats, creating long, durable polymer chains.
Some common products of condensation polymerisation include polyesters (used in fabrics and bottles) and polyamides (used in clothing and engineering materials). These polymers are not only essential for GCSE Chemistry but also have significant real-world applications, from making plastics to creating strong, lightweight materials like nylon.
Understanding what condensation polymerisation is helps you appreciate how materials around us are made, whether it’s the plastic in bottles or the fibers in clothing.
How Does Polymerisation Work?
To understand how materials like plastics or fibers are made, it’s important to know how polymerisation works. Polymerisation is the process where small molecules, called monomers, join together to form long chains called polymers. These polymers are the building blocks of many everyday materials, from plastic bottles to fabrics.
In condensation polymerisation, the process works a bit differently. Monomers must have two functional groups, such as carboxylic acids, alcohols, or amines, which can react with each other. This reaction not only forms bonds between the monomers but also releases a small molecule, like water or methanol, each time a bond is created.
Here’s a step-by-step breakdown of how condensation polymerisation works:
- Monomers React: Monomers with functional groups, like a dicarboxylic acid and a diol, come into contact.
- Bond Formation: The functional groups react chemically, creating strong bonds that link the monomers together.
- Small Molecule Release: With each reaction, a small molecule, such as water, is released as a byproduct.
This process repeats over and over, forming long, durable polymer chains. For example, in the production of polyesters, a dicarboxylic acid reacts with a diol to create the polymer, with water being released. Similarly, nylon, a type of polyamide, is formed by reacting a diamine with a dicarboxylic acid, also producing water.
The key requirements for this process:
- Monomers with two reactive functional groups.
- Specific conditions, like the use of catalysts and heat, to speed up the reaction.
By understanding the condensation polymerisation reaction, you can see how simple molecules combine to create complex, useful materials. This process explains the science behind the creation of plastics, fabrics, and even biological polymers like proteins.
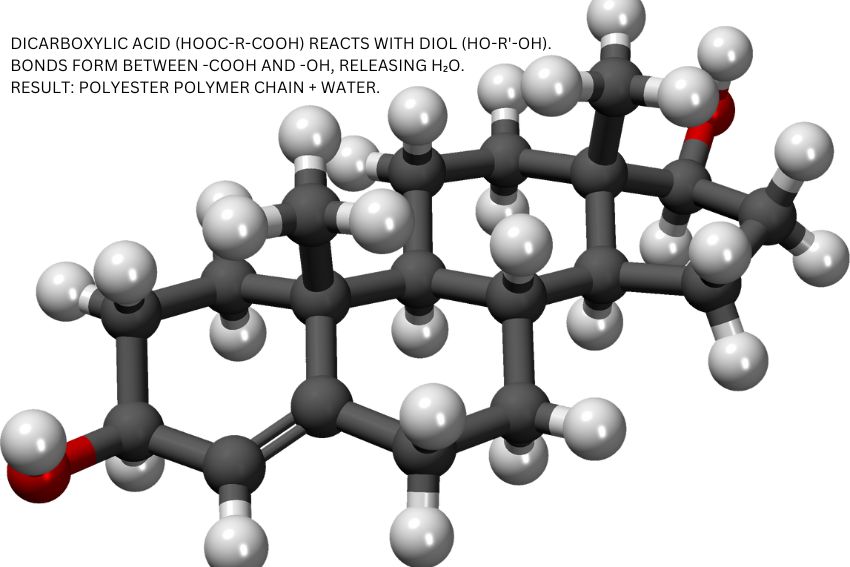
Condensation Polymerisation in GCSE Chemistry
Condensation polymerisation is a key topic in GCSE Chemistry because it explains how many important materials, both synthetic and biological, are made. It’s not just about plastics like nylon or polyester, it’s also the process behind the formation of biological molecules that are essential for life.
A common question you might encounter in exams is:
“What is produced when two amino acids react together by condensation polymerisation?”
The answer is simple: A dipeptide is formed, along with a molecule of water. This is because the amino group (-NH₂) of one amino acid reacts with the carboxyl group (-COOH) of another amino acid, forming a peptide bond.
In biology, proteins are created through a series of condensation reactions, where many amino acids link together to form long chains called polypeptides. Similarly, carbohydrates like starch and cellulose are also formed through condensation polymerisation, where simple sugar molecules join together.
In GCSE Chemistry, this topic not only helps you understand the chemistry behind synthetic materials like plastics but also provides insight into the natural processes that create essential biological polymers. By learning about condensation polymerisation, you’re exploring the foundation of both the materials we use and the molecules that sustain life.
Real-Life Applications of Condensation Polymerisation
Now that you understand how this chemical reaction works, let’s explore its incredible real-life applications. This process is at the heart of creating both synthetic materials, like plastics, and natural polymers, like proteins and carbohydrates.
One of the most important uses of condensation polymerisation is in plastic production. Take polyesters, for example. Polyethylene terephthalate (PET) is a common polyester made through condensation polymerisation. It’s widely used in making plastic bottles, food containers, and even fabrics like polyester clothing.
Another group of plastics formed through this process are polyamides, which include materials like nylon and Kevlar. Nylon is commonly used in clothing, ropes, and toothbrush bristles, while Kevlar is known for its strength and is used in bulletproof vests and industrial applications.
Condensation polymerisation also plays a vital role in biology. Proteins, which are made of amino acids, and polysaccharides, such as starch and cellulose, are examples of natural polymers formed by this process. These biological polymers are essential for life, as they provide structure, store energy, and perform countless functions in the body.
Whether it’s the plastic in your water bottle or the protein in your body, condensation polymerisation is the reason these materials exist. Understanding this process helps us see how chemistry shapes both the world around us and the life within us.

GCSE Chemistry: How Is Plastic Made?
Now that we’ve explored the basics of condensation polymerisation, let’s dive into how it’s used to make plastics. This process is not only crucial in industrial manufacturing but also an important topic in GCSE Chemistry, helping students understand how modern materials are created.
Here’s how plastic is made through condensation polymerisation:
- Selecting Monomers: The process begins by choosing monomers with two functional groups. For example, polyesters are made from diols (a type of alcohol) and dicarboxylic acids, while polyamides like nylon use diamines and dicarboxylic acids.
- Polymerisation Reaction: These monomers undergo condensation polymerisation, forming long polymer chains while releasing small molecules like water as byproducts. This reaction is a core concept in condensation polymerisation GCSE lessons.
- Shaping and Cooling: Once the polymer is formed, it’s shaped into products like fibers, sheets, or pellets. After cooling, it solidifies into final items such as bottles, ropes, or packaging materials.
This process explains how common items like PET bottles and nylon ropes are created. It also helps answer questions like, “Where does plastic come from?” and “How is plastic made?” By linking these everyday materials to their origins in polymerisation, you can better appreciate how science shapes the world around us.
The Impact of Condensation Polymerisation: Benefits and Drawbacks
Condensation polymerisation is a powerful process that has transformed industries and everyday life. However, like most things, it comes with both advantages and drawbacks. Let’s explore them.
Benefits
- Strong and Durable Polymers: The polymers produced through condensation polymerisation, such as nylon and PET, are incredibly strong and long-lasting. This makes them perfect for products like ropes, fabrics, and bottles that need to withstand wear and tear.
- Wide Variety of Uses: From clothing and packaging to engineering materials, condensation polymerisation has endless applications. It’s used to create materials we rely on daily, such as polyester clothing, water bottles, and even industrial-grade plastics.
Limitations
- Non-Biodegradable Plastics: A major drawback of synthetic polymers created through condensation polymerisation reaction is that many are non-biodegradable. This contributes to environmental pollution and waste buildup, making it a significant global concern.
- Precise Conditions Needed: The process requires very specific conditions, including the right functional groups, high temperatures, and often catalysts. This complexity can make production expensive and energy-intensive.
In summary, while condensation polymerisation gives us versatile and durable materials, it also poses environmental challenges that scientists and industries are working hard to address.
Conclusion
In conclusion, condensation polymerisation is a vital process in both chemistry and everyday life. It’s the science behind creating strong, durable materials like plastics and nylon, and it also explains how essential biological polymers like proteins and carbohydrates are formed.
For students studying condensation polymerisation GCSE, this topic is especially important for understanding plastic production, polymerisation reactions, and the role of chemistry in shaping the modern world. From answering questions like “How is plastic made?” to exploring “What is condensation polymerisation?”, this process connects classroom learning to real-world applications.
If you’re finding this topic challenging, consider seeking extra help from online GCSE Chemistry tutors. They can provide personalized guidance, clarify difficult concepts, and help you feel confident in tackling exam questions.
Take the time to dive deeper into polymerisation, whether it’s for your exams or simply to understand the science behind everyday materials. The more you explore, the more you’ll appreciate how chemistry helps us build and sustain our world.
FAQs:
What is condensation polymerisation?
Condensation polymerisation is a chemical reaction where monomers with two functional groups join to form polymers, releasing small molecules like water as byproducts.
How does polymerisation work?
Polymerisation works by joining small molecules, called monomers, to form long chains called polymers. In condensation polymerisation, monomers with functional groups react to form bonds, releasing small byproducts like water.
What is produced when 2 amino acids react together by condensation polymerisation?
When two amino acids react, they form a dipeptide and a molecule of water through the formation of a peptide bond.
There are two types of polymerisation. Condensation is one. What is the other?
The other type of polymerisation is addition polymerisation, where monomers join to form polymers without releasing byproducts.
Condensation polymerisation involves monomers that have how many functional groups?
Condensation polymerisation involves monomers with two functional groups, which react to form long polymer chains.
Complete the following sentence. In condensation polymerisation, two __________ monomers join.
In condensation polymerisation, two functional group-containing monomers join.