A-Level Periodic Table Explained
Understanding the Periodic Table
The Periodic Table stands as a fundamental resource in the field of chemistry, serving as a chart that systematically organises all known elements based on their properties. First introduced by Dmitri Mendeleev in 1869, it has evolved to include the latest discoveries and research. Despite these updates, the periodic table used in GCSE and A-level chemistry AQA courses remains quite similar to the traditional version, maintaining its essential structure and purpose.
AQA A level periodic table arranges elements by their atomic number and chemical properties. Each entry includes the element’s atomic number, atomic mass, and electron configuration, as well as information on valency, oxidation state, and group/period positioning. Understanding the periodic table is crucial for predicting how elements interact, form compounds, and behave chemically.
At the top of the table, you’ll find elements like hydrogen, which constitutes about 90% of atoms. As you move down, you’ll encounter elements with higher atomic numbers, such as Oganesson. The atomic number indicates the number of protons in an element’s nucleus, with electrons orbiting around this nucleus.
Layout Specific to AQA A level periodic table
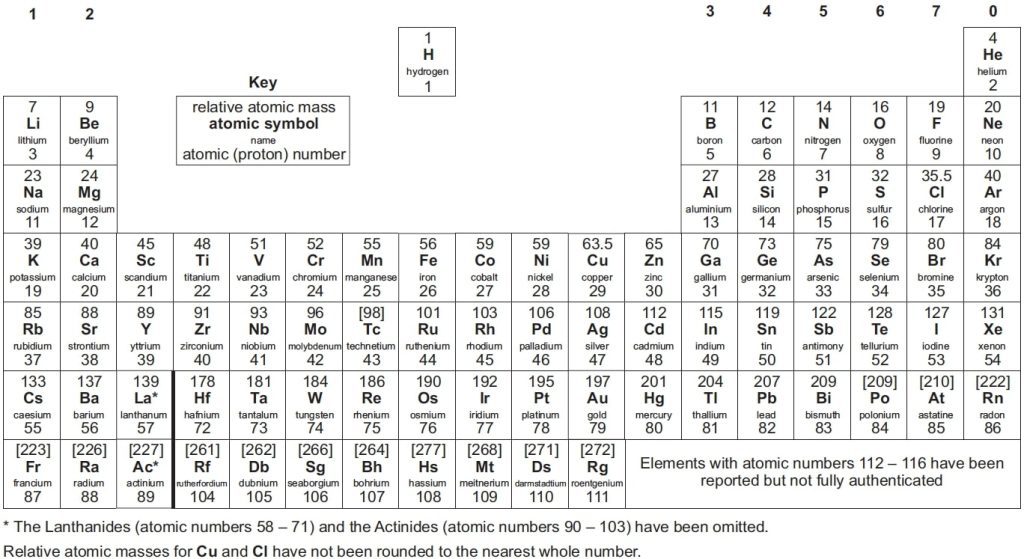
The layout of the A-level periodic table mirrors that of other periodic tables, including those used at the GCSE. The periodic table is divided into vertical columns known as groups and horizontal rows known as periods.
What are Groups in A-Level Chemistry?
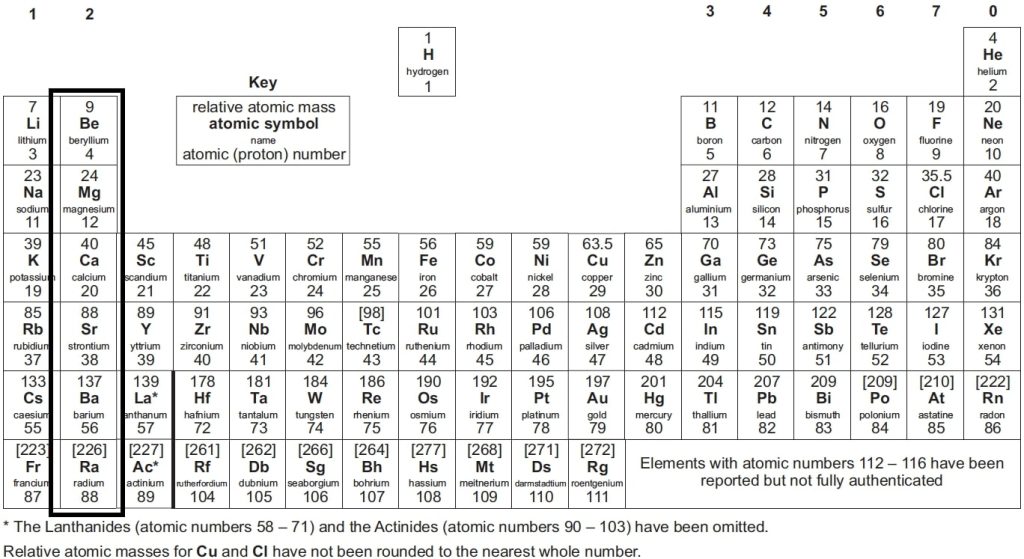
Elements in the same group share similar properties. For instance, Group 1 elements, known as alkali metals (e.g., lithium, sodium, potassium), are highly reactive. This shared reactivity is due to each element having the same number of electrons in its outermost shell.
Key Points:
- Elements in the same group exhibit similar chemical properties.
- Group members have the same number of outer shell electrons.
What are Periods in AQA A level Chemistry?
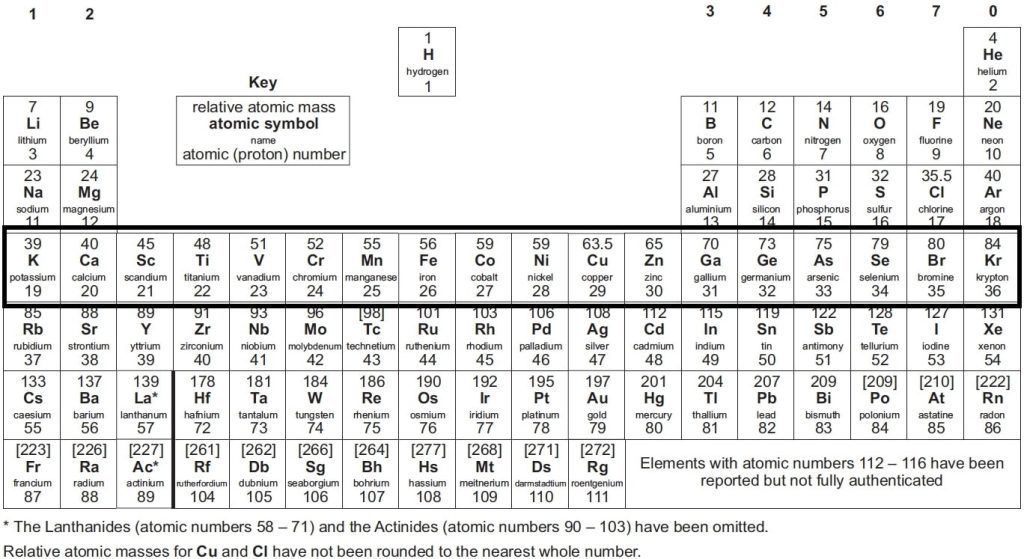
Elements are also organised into periods, which are horizontal rows on the table. Each period number signifies the number of atomic shells an element possesses. For example, lithium, located in period 2, has two electron shells.
Key Points:
- Periods indicate the number of electron shells.
- Elements in the same period have progressively increasing atomic numbers and electron shells.
Do You Need to Know the AQA A Level Periodic Table by Heart?
While memorising the entire periodic table is unnecessary, understanding how to read and interpret it is essential for A-Level chemistry. Knowing the characteristics of elements in specific groups or periods and the general layout will aid significantly in exams.
During A-Level chemistry exams, students are provided with a copy of the periodic table, so there’s no need to memorise it by heart. Familiarity with the table’s layout and the ability to quickly locate information is crucial.
Fun Facts About Chemistry
Chemistry is full of intriguing facts that can make learning more enjoyable:
- Mpemba Effect – Hot water can freeze faster than cold water.
- Water and Salt – Adding salt to a full glass of water makes the water level drop.
- Mixing Volumes – Mixing equal volumes of alcohol and water results in a total volume less than the sum of the individual volumes.
- Letter J – The periodic table does not include the letter J.
- Capsaicin – Birds are immune to the heat from hot peppers, unlike humans.
- Water Toxicity – Drinking excessive amounts of water can be fatal.
- Glass – Technically, glass is a liquid that flows very slowly.
- DNA – DNA acts as a flame retardant.
- Temperature Perception – Water feels colder than air at the same temperature.
- Molecular Structures – A car tyre is essentially one large molecule.
Conclusion
A-Level Chemistry is challenging but rewarding, with the AQA A level periodic table at its core. While it’s considered more demanding than some of the easiest A-level options, understanding this crucial tool not only aids in exams but also lays the groundwork for future scientific endeavours. With the help of a dedicated tutor, mastering the periodic table and other chemistry concepts can become a more manageable and enjoyable journey. Who knows, your exploration of the periodic table could inspire you to contribute to the discovery of the next element!
If you are searching for A-Level Chemistry tutors, consider using Edumentros. This online tutoring platform connects students and parents with tutors from top UK universities, ensuring high-quality educational support tailored to individual needs.
FAQs:
Do you get a periodic table in Chemistry A-level?
Yes, A-Level Chemistry students typically receive a periodic table during exams. The table provided is a standard one, containing essential information like element symbols, atomic numbers, and relative atomic masses, but without additional details like trends or group characteristics. Familiarity with the layout and key trends in the periodic table is still important, as understanding these patterns can help with questions on properties and reactivity.
What are the periodic trends in A-level Chemistry?
In A-Level Chemistry, students explore several key periodic trends that help explain element behaviour and reactivity across the periodic table. These trends include:
- Reactivity: Metals tend to be more reactive as you move down a group due to lower ionisation energy. For non-metals, reactivity often decreases down a group as electronegativity decreases (e.g., halogens).
- Atomic Radius: Generally decreases across a period (left to right) due to an increasing nuclear charge that pulls electrons closer to the nucleus. Atomic radius increases down a group as more electron shells are added.
- Ionisation Energy: The energy needed to remove an electron from an atom. It generally increases across a period because of a stronger attraction between the nucleus and the outer electrons. However, it decreases down a group as outer electrons are farther from the nucleus and more shielded by inner electrons.
- Electronegativity: A measure of an atom’s ability to attract electrons in a bond. Electronegativity increases across a period as nuclear charge increases, but decreases down a group due to greater atomic radius and shielding effect.
- Metallic Character: Decreases across a period as elements move from metals to non-metals. It increases down a group as atoms lose electrons more easily due to lower ionisation energies.
- Melting and Boiling Points: Vary depending on the type of bonding. Metals (on the left) generally have higher melting points due to metallic bonding. Non-metals show a range of melting points; for example, noble gases have low boiling points, while network covalent structures like silicon have high melting points.
- Reactivity: Metals tend to be more reactive as you move down a group due to lower ionisation energy. For non-metals, reactivity often decreases down a group as electronegativity decreases (e.g., halogens).
What grade level do you learn the periodic table?
Students typically begin learning the basics of the periodic table around Key Stage 3 (ages 11-14), usually in Year 7 or Year 8 in the UK. At this stage, they cover foundational concepts, such as element symbols, atomic numbers, and simple group characteristics (like metals and non-metals).
A more in-depth study of the periodic table, including periodic trends, atomic structure, and bonding, typically occurs in GCSE Chemistry (Key Stage 4, ages 14-16). Here, students learn to interpret the table in terms of electron configuration, reactivity, and the properties of different groups, like alkali metals and halogens.
In A-Level Chemistry (Key Stage 5, ages 16-18), students explore the periodic table at a much deeper level, focusing on advanced concepts like ionisation energies, transition metals, periodic trends, and complex bonding theories, building on the foundations learned at GCSE.
Can I get an A * in A-level chemistry?
Yes, achieving an A* in A-Level Chemistry is possible with consistent effort, effective study strategies, and a thorough understanding of the material. Here are some key tips to help you reach this goal:
- Master the Basics: Make sure you have a solid understanding of fundamental concepts from GCSE, as A-Level builds on this foundation, especially in areas like atomic structure, bonding, and the periodic table.
- Understand, Don’t Memorise: Focus on truly understanding the principles rather than rote memorisation. Chemistry often requires applying knowledge to unfamiliar scenarios, so a deep comprehension is crucial.
- Practice Past Papers: Work through as many past papers as possible under timed conditions. This will familiarise you with the exam format, question types, and marking schemes. Aim to understand why each answer is correct.
- Revise Effectively: Create a revision plan, covering each topic thoroughly. Use varied resources such as textbooks, online tutorials, and revision guides. Make concise notes, diagrams, and flashcards for quick review.
- Focus on Weak Areas: Regularly identify topics you find challenging and dedicate extra time to them. Seeking help from a teacher, tutor, or peer can also be very beneficial.
- Practise Calculations and Equations: Many A-Level Chemistry questions involve calculations (moles, titrations, enthalpy changes). Practise these consistently to build speed and accuracy.
- Understand Practical Skills: The practical component of A-Level Chemistry is important. Familiarise yourself with the required practicals and understand the methods, observations, and outcomes, as these often appear in written exams.
- Stay Organised and Manage Time Well: Effective time management is crucial, both for study schedules and during exams. Break down topics into manageable sections and tackle them consistently.
Achieving an A* is challenging, but with dedication, resilience, and effective study habits, it’s definitely attainable!
Which Periodic Table trends should I revise for AQA A-Level?
When you revise the AQA A Level periodic table content, focus on the major trends: atomic radius, ionisation energy, and electronegativity. These patterns help you explain why elements behave the way they do. You should also look at melting and boiling point trends across Period 3, plus changes in reactivity in Group 2 and the halogens, as these are tested often in AQA exam questions.