Table of Contents:
Periodic Table – What is it?
The periodic table is considered one of the most important tools in chemistry. It’s a chart that organises all of the elements according to their properties, and it’s been used for over a century by chemists around the globe. It was first developed by Dmitri Mendeleev in 1869 and has been updated over time to reflect new research and discoveries about elements. The periodic table has been around for over 150 years, and is still used in GCSE and A-level chemistry courses to this day. It’s important to mention that A-level and GCSE periodic tables are not that different from the typical periodic table. 🧪
The periodic table is organised by atomic number and chemical properties. It contains all the known elements, including their atomic numbers, atomic mass and electron configurations. Additionally, it shows their chemical properties such as valency, oxidation state and their position in groups or periods.
The periodic table helps you understand how different elements interact with each other, which ones are likely to form compounds together, and how those compounds might behave when you try to make them.
The periodic table presents a list of all known elements arranged in order of their atomic number—from hydrogen, which makes up 90% of atoms, to Oganesson (which has recently been discovered). The atomic number tells you how many protons are in each atom of that element. Protons are positively charged particles that make up an atom’s nucleus, while electrons orbit around the nucleus of an atom.
The Layout of the Periodic Table GCSE
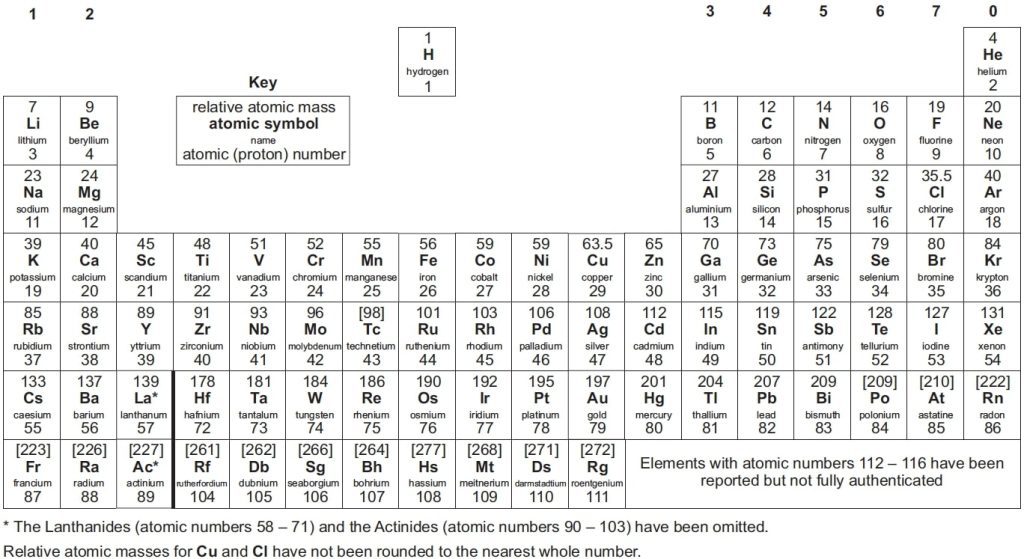
GCSE periodic table’s layout is the same as almost all other periodic tables including A-level periodic table.
What are Groups in GCSE Chemistry?
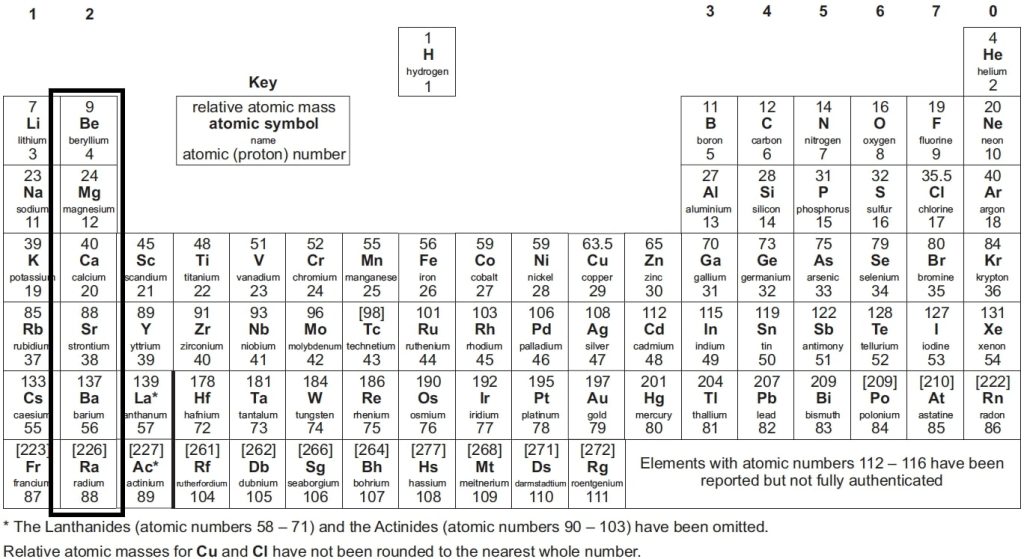
📌 The elements are arranged in vertical columns known as groups – these groups contain the same types of properties.
📌 The members of each group share a number of common characteristics. The alkali metals in group 1, such as lithium, sodium and potassium are all very reactive.
📌 Elements in a group have the same chemical properties because they all have the same number of electrons in their outermost shell.
TLDR:
📌 Elements in each group of the periodic table have a distinct set of properties and react in similar ways. Each element has its own number of electrons, which determines how it reacts when combined with other elements.
What are Periods in GCSE Chemistry?
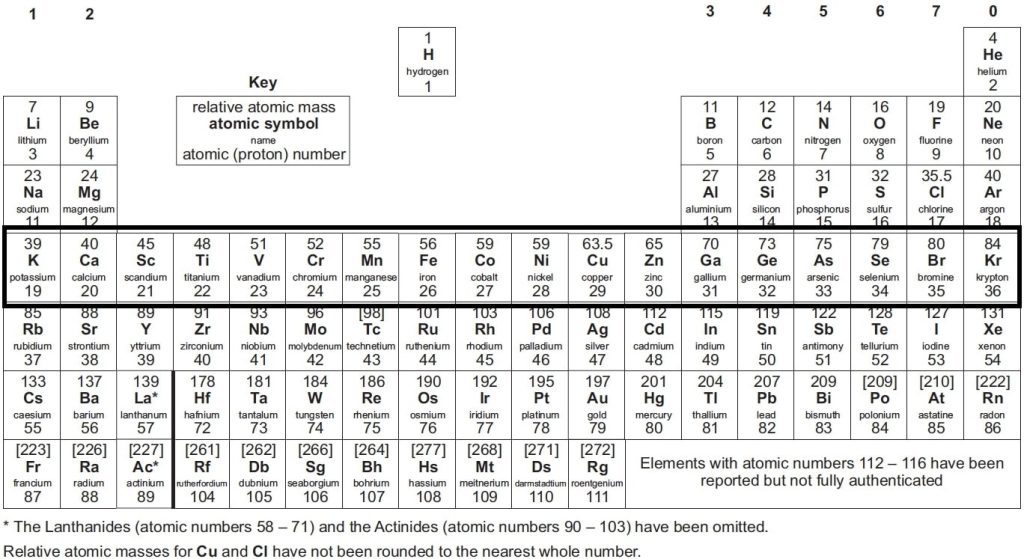
📌 Elements on the periodic table are also arranged in horizontal rows. The table is divided into periods—groups of elements with similar properties—and each period has a number representing its position on the chart, from 1 (the first) to 7 (the last).
📌 Each period in the table contains a certain number of atomic shells. For example, lithium is in period 2 and has two shells.
TLDR:
📌 The group number indicates the number of electrons in the outer shell.
📌 There are horizontal rows known as periods, which tell you the number of electron shells that an element has.
Do You Need to Know the GCSE Periodic Table by Heart?
The short answer is no, you absolutely do NOT need to know the GCSE periodic table by heart.
Even the most professional and experienced Chemists don’t know it by heart. However, you still have to know how to read your GCSE periodic table (or A-level periodic table). You have to be able to understand how the elements in one group or period relate to each other and what characteristics they have in common. Also, you have to know which part of the periodic table contains elements such as noble gases, nonmetals, transition metals or halogens.
The best way to remember those kinds of things is to use the periodic table regularly. You can also use online websites such as Ptable.
Is The Periodic Table allowed on the GCSE Chemistry Exam?
Well, you can’t take your own – BUT, worry not! They will give you a fresh copy when you get to the exam! 📃
Fun Facts About Chemistry

Now that the explanation is out of the way, here are some fun facts about chemistry:
💡 Hot water freezes faster than cold water! – this is called the Mpemba effect.
💡 If you pour a handful of salt into a full glass of water, the water level will actually go down.
💡 If you mix half a litre of alcohol and half a litre of water, the total volume of the liquid will not be equal to one litre. It will be less!
💡 The only letter not appearing on the periodic table is J.
💡 Birds are immune to the burning sensation of hot peppers. Pepper gets this heat from a molecule called Capsaicin and whilst it’s irritant to humans, birds are immune to it!
💡 It’s possible to die from drinking too much water.
💡 Glass is actually a liquid which flows very very slow one…
💡 DNA is a flame retardant.
💡 Even if water and air are the same temperatures, water will still feel colder.
💡 A car tire is one large molecule.
To Sum Up
GCSE Chemistry is one of the toughest GCSE courses and many students struggle to achieve their desired score. There are lots of complex problems to understand and even more to remember by heart. This is why students usually dread Chemistry, especially if they know they need to study it all on their own.
However, studying chemistry does not have to be that difficult at all. It can be much more enjoyable if you’re receiving support from a GCSE chemistry tutor who has been in your shoes and succeeded. On the positive side, GCSE chemistry gives you the opportunity to have a rewarding and high-paid career path and you may well become the next well-known chemist! Scientists around the world are looking to invent the 119th element and who knows, it may well be you!
Most importantly, don’t stress and remember, there’s always a smarter way out! 💪