What Is Volume Of Gas? – Equation, laws and examples For GCSE Chemistry
GCSE Chemistry offers students the foundational knowledge they need to understand the world around them, and one of the important concepts covered in this subject is the volume of gas. Understanding how gases behave, how their volume is measured, and how it relates to pressure and temperature is essential not only for GCSE exams but also for real-life applications in science and industry. The volume of gas equation plays a key role in this area, as it allows students to calculate and predict the behaviour of gases under different conditions.
This blog aims to guide students through the essential concepts surrounding the volume of gas, breaking down the equation and its applications in simple, clear terms. We will explain the key principles, offer tips on how to apply the gas laws in practical scenarios, and provide helpful revision strategies to ensure students feel confident in mastering this important topic for their exams.
What is the Volume of Gas?
The volume of gas refers to the amount of space a gas occupies. This can be influenced by various factors, including temperature, pressure, and the number of gas particles. Unlike solids and liquids, gases have particles that are far apart and move freely, meaning they can easily expand or contract depending on the conditions.
The relationship between the volume of a gas, pressure, and temperature is governed by a series of gas laws. These include Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law, which are all used to describe how gas volume changes under different conditions. The Ideal Gas Law combines these individual laws and provides a more general equation that relates pressure, volume, and temperature.
What Is Volume Of Gas Equation?
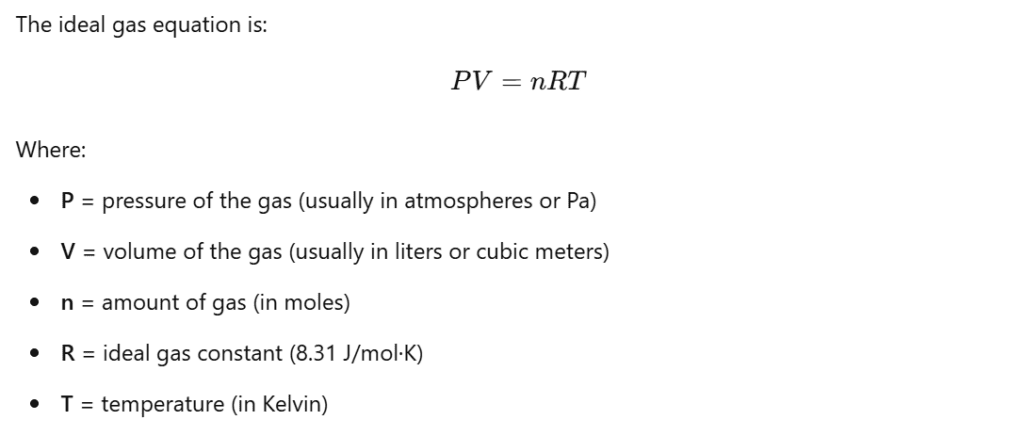
This equation helps to predict how gases will behave when changes in pressure, temperature, or volume occur. By understanding this, students can calculate the volume of gas in different conditions, which is critical in both theoretical problems and practical experiments.
Key Points to Remember:
- Temperature must always be in Kelvin for gas law calculations. To convert from Celsius to Kelvin, simply add 273.15.
- Pressure and volume are inversely related according to Boyle’s Law; as one increases, the other decreases, provided temperature and the amount of gas remain constant.
- Charles’ Law shows that volume is directly proportional to temperature when pressure and amount of gas are constant. In other words, as temperature increases, volume also increases.
- Gay-Lussac’s Law states that for a fixed volume, the pressure of a gas is directly proportional to its temperature.
What is the Molar Volume of a Gas?
The molar volume of a gas is the volume occupied by one mole of gas at a specific temperature and pressure, typically at standard conditions (STP: 0°C and 1 atmosphere of pressure). The molar volume is an important concept in chemistry as it helps relate the amount of gas to its volume under known conditions.
At standard temperature and pressure (STP), the molar volume of an ideal gas is approximately 22.4 liters. This means that one mole of any ideal gas will occupy a volume of 22.4 L when measured at STP.
Formula for Molar Volume:
The molar volume (V_m) can be calculated using the ideal gas equation:Vm=VnV_m = \frac{V}{n}Vm=nV
Where:
Vm = V / n
- V is the volume of the gas,
- n is the number of moles of gas.
For gases under standard conditions, we often use the known value of 22.4 L per mole at STP to make calculations easier.
Example: Calculate the volume of 2 moles of an ideal gas at 298 K and 1 atm pressure.
Given:
n = 2 mol
T = 298 K
P = 1 atm
R = 0.0821 L·atm/(mol·K)
Ideal Gas Equation: PV = nRT
Rearrange to find volume: V = (nRT) / P
Calculation:
V = (2 mol × 0.0821 L·atm/mol·K × 298 K) / 1 atm
V = 48.9 L
Answer: The volume of the gas is 48.9 liters.
Example: Calculate the volume change of a gas when its pressure and temperature change.
Given:
P1 = 1.0 atm
V1 = 5.0 L
T1 = 300 K
P2 = 2.0 atm
T2 = 350 K
Ideal Gas Law Equation:
(P1 V1) / T1 = (P2 V2) / T2
Rearrange to solve for V2:
V2 = (P1 V1 T2) / (P2 T1)
Calculation:
V2 = (1.0 atm × 5.0 L × 350 K) / (2.0 atm × 300 K)
V2 = 1750 L·K / 600 atm·K
V2 = 2.92 L
Answer: The new volume of the gas is 2.92 L.
Conclusion
Understanding the volume of gas equation is essential for mastering the concepts of gas behaviour in GCSE and A Level Chemistry. By applying this equation, students can solve problems related to pressure, temperature, and volume changes, providing a solid foundation for further study in chemistry.
For students looking for additional support, online GCSE chemistry tutors can offer personalised guidance, helping to clarify complex concepts and refine problem-solving techniques. With expert assistance, students can build confidence, improve their understanding, and approach their exams with the knowledge needed to succeed.
Other Topics Related to GCSE Chemistry:
How To Calculate Relative Atomic Mass? GCSE Chemistry Guide
GCSE Chemistry – Bonding, Structure, and the Properties of Matter
FAQ: Volume Of Gas
What is Volume?
Volume refers to the amount of space that a substance or object occupies. In the context of gases, it is the amount of space a gas occupies within a container. The volume of a gas depends on factors such as pressure, temperature, and the number of gas molecules present.
What is a Mole?
A mole is a unit used in chemistry to express the amount of a substance. One mole is equivalent to 6.022 × 10²³ (Avogadro’s number) particles, atoms, or molecules. The mole allows chemists to count and measure quantities of substances in a manageable way, connecting the microscopic world of atoms and molecules with macroscopic measurements like grams or liters.
What is the relationship between Volume and Moles?
The volume of a gas is directly proportional to the number of moles of gas, assuming pressure and temperature are constant. This relationship is described by Avogadro’s Law, which states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules. Thus, if the number of moles increases, the volume will also increase.
What is the unit of the volume of a gas?
The unit of the volume of a gas is typically expressed in liters (L) or cubic meters (m³). When using the Ideal Gas Law, volume is commonly measured in liters for ease of calculation, especially in laboratory conditions.