In chemistry, enthalpy is a way to measure energy changes during reactions. One important concept is the enthalpy of hydration – the energy change that happens when gaseous ions dissolve in water. It plays an important role in understanding why some substances dissolve easily while others do not, and why certain reactions give off or absorb heat.
Understanding the enthalpy change of hydration isn’t just helpful for exams, it explains real world processes like salt dissolving in water or how ionic compounds behave in solution.
In this guide, we’ll break down what the enthalpy of hydration really means. You’ll learn how it works, what affects it, and how to calculate it using simple methods. We’ll also look at lattice enthalpy, solution equations, and common mistakes to avoid. Let’s dive in and make this topic easy to understand one step at a time.
- Enthalpy of Hydration Definition – A Level Chemistry
- The Hydration Process Explained
- Factors Affecting Enthalpy of Hydration – A Level Chemistry
- Lattice Enthalpy and Enthalpy of Solution – A Level Chemistry
- Enthalpy of Hydration Formula and Calculations
- Enthalpy of Hydration – Common Misconceptions to Avoid
- A Level Chemistry Study Resources
- Conclusion
Enthalpy of Hydration Definition – A Level Chemistry
The enthalpy of hydration is the energy released when one mole of gaseous ions dissolves in water and forms hydrated ions in a very dilute solution. In simple terms, it’s the heat given off when ions are surrounded by water molecules.
This process is always exothermic, which means it gives out energy. That’s because water molecules are attracted to the charged ions. As they surround and bond with them, energy is released.
You might not notice it in your daily life, but hydration happens all around you. For example, when certain salts dissolve in water, the solution warms up, that’s the enthalpy of hydration at work. It’s also important in biological systems, where ions like sodium and potassium need to dissolve in water to move through cells. Understanding this concept helps explain why some substances dissolve better than others and why some solutions release heat while others absorb it.
The Hydration Process Explained
Now that you know what the enthalpy of hydration means, let’s look at what actually happens during the process. When an ionic compound dissolves in water, it breaks apart into separate ions – for example, NaCl splits into Na⁺ and Cl⁻. These ions were originally held together by strong forces in the solid crystal. Once they’re free in the gas phase, water molecules begin to surround them. This surrounding process is called hydration.
Water is a polar molecule, which means it has a slightly positive and a slightly negative side. The oppositely charged parts of the water molecules are attracted to the ions. As they move in and attach to the ions, they form ion-dipole bonds, and this interaction releases energy. That’s why hydration is an exothermic process.
If you visualise this at the particle level, imagine water molecules rushing in and wrapping themselves around each ion like a tiny magnetic cloud. The stronger the attraction between the ion and water, the more energy is released and the more negative the enthalpy change of hydration becomes.
Factors Affecting Enthalpy of Hydration – A Level Chemistry
Not all ions release the same amount of energy when they’re hydrated. The enthalpy of hydration depends on two main things: ionic charge and ionic radius.
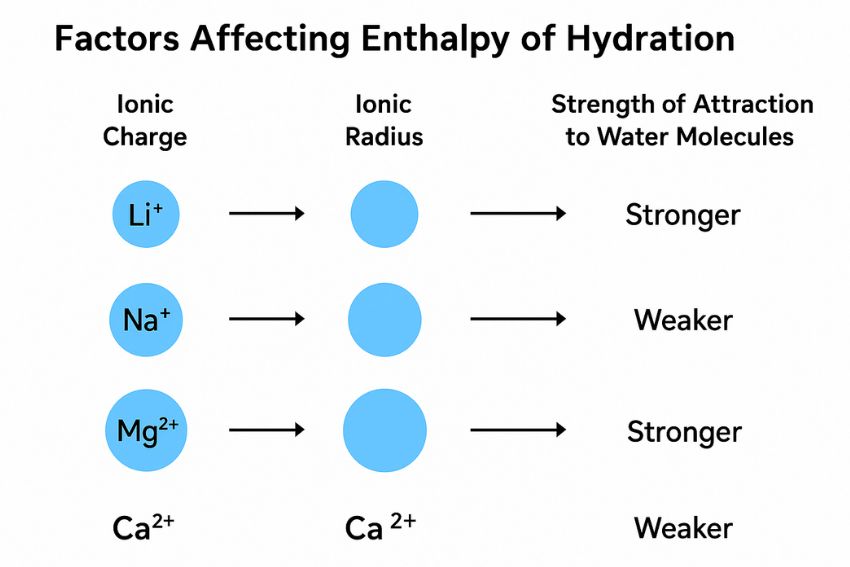
Ions with a higher charge attract water molecules more strongly. This means more energy is released when they’re hydrated. For example, Mg²⁺ has a charge of +2, while Na⁺ only has a +1 charge. As a result, the hydration enthalpy of Mg²⁺ is more negative, it releases more energy during hydration.
Ionic size matters too. Smaller ions have a higher charge density, meaning the same charge is packed into a smaller space. This leads to stronger attraction between the ion and water molecules. Compare Li⁺ and Na⁺: both have a +1 charge, but Li⁺ is smaller, so it has a more exothermic (more negative) enthalpy of hydration.
These factors also affect solubility. When hydration energy is high enough to overcome the forces holding the solid together (the lattice energy), the substance dissolves more easily. That’s why understanding these are important, it helps predict which salts dissolve well in water and which ones don’t.
Lattice Enthalpy and Enthalpy of Solution – A Level Chemistry
To fully understand the enthalpy of hydration, it helps to look at how it connects with two other key ideas lattice enthalpy and the enthalpy of solution.
Lattice enthalpy is the energy needed to break an ionic solid into its separate gaseous ions. This step is always endothermic because you have to put in energy to pull the ions apart from their tightly packed structure.
Once the ions are free, they dissolve in water and release energy, that’s where hydration enthalpy comes in. So when an ionic compound dissolves in water, two energy changes are happening: breaking the lattice (which takes in energy) and hydrating the ions (which gives out energy). These changes are linked in the enthalpy of solution equation: Enthalpy of solution = Lattice enthalpy + Enthalpy of hydration.
If the hydration enthalpy is more negative than the lattice enthalpy is positive, the overall process releases energy, making the compound more likely to dissolve. But if the lattice enthalpy is too large, the substance may stay solid, even if hydration is favourable. This balance helps explain why some salts dissolve easily in water, while others don’t.
Enthalpy of Hydration Formula and Calculations
Once you know what enthalpy of hydration is, the next step is learning how to calculate it. This is where Hess’s Law becomes really useful. It allows you to work out unknown energy values by using known ones from a simple enthalpy cycle. There’s no single fixed enthalpy of hydration formula, but the one commonly used in these questions is:
Enthalpy of hydration = Enthalpy of solution – Lattice enthalpy
This equation helps you find the hydration enthalpy if you’re given the other two values. You’ll often see this in exam questions where one value is missing and you’re asked to fill in the gap. Let’s go through a clear example:
Enthalpy of solution of potassium chloride (KCl) = +18.6 kJ/mol
Lattice enthalpy of KCl = +701 kJ/mol
Now plug it into the formula:
Enthalpy of hydration = 18.6 – 701 = -682.4 kJ/mol
The negative sign tells you this is an exothermic process, energy is released when the ions are hydrated. To prepare for exams, practise with different sets of data and get used to rearranging the formula. It’s a great way to build your confidence with energy calculations.
Enthalpy of Hydration – Common Misconceptions to Avoid
As you revise enthalpy of hydration, it’s easy to get caught out by a few tricky points. Let’s clear up the most common mistakes students make.
First, many learners get confused about the sign of hydration enthalpy. Since energy is released when ions are surrounded by water, the value is always negative. If you ever see a positive value, it’s likely a mistake or from a different step in the energy cycle.
Another common mix-up is between hydration enthalpy and enthalpy of solution. Remember: hydration refers only to the energy released when water surrounds the ions. Enthalpy of solution includes both hydration and lattice energy. It tells you the overall energy change when a solid dissolves in water.
Finally, the phrase “infinitely dilute solution” can sound confusing. It simply means that there’s enough water for the ions to be completely surrounded, with no interactions between them. It’s a way to make sure the measurement reflects just the hydration process, without interference. Understanding these details will help you answer questions more confidently and avoid these common mistakes.
A Level Chemistry Study Resources
Revising topics like enthalpy of hydration is much easier when you have the right resources. Whether you’re looking for clear notes, practise questions, or video explanations, these trusted platforms can help you stay on track.
Chemguide: A great site for deeper explanations of hydration, lattice enthalpy, and Hess’s Law. Ideal if you want to really understand how and why things work.
Royal Society of Chemistry: Education Resources: Features high-quality teaching and learning materials, videos, and articles which are useful for you.
BBC Bitesize – You can find lots of interesting information about A Level Chemistry itself.
Revision Science – Here you can see the past papers of A Level Chemistry which is useful for the revision.
Conclusion
Understanding the enthalpy of hydration is more than just memorising definitions, it’s about making sense of how energy changes happen in real chemical reactions. In this guide, we covered what hydration enthalpy means, how to calculate it, what affects it, and how it connects to other key ideas like lattice enthalpy and enthalpy of solution.
These concepts are essential for A Level Chemistry and beyond. They often appear in exam questions, especially those involving Hess’s Law or solubility trends. The better you understand them, the more confidently you’ll handle calculations and explain energy changes in your answers. If you want to get even better, keep practising with past paper questions and challenge yourself to apply what you’ve learnt in different scenarios.
And if you ever feel stuck or want extra help, don’t worry – you’re not alone. If you’re looking for online tutoring in A Level Chemistry, working with a tutor can make all the difference. A good tutor can explain difficult topics in a clear way and help you stay on track.
You Might Find This Interesting
A Level Chemistry – Everything You Need to Know
A Level Periodic Table Explained