Understanding the Periodic Table
The Periodic Table stands as a fundamental resource in the field of chemistry, serving as a chart that systematically organises all known elements based on their properties. First introduced by Dmitri Mendeleev in 1869, it has evolved to include the latest discoveries and research. Despite these updates, the periodic table used in GCSE and A-level chemistry courses remains quite similar to the traditional version, maintaining its essential structure and purpose.
The periodic table arranges elements by their atomic number and chemical properties. Each entry includes the element’s atomic number, atomic mass, and electron configuration, as well as information on valency, oxidation state, and group/period positioning. Understanding the periodic table is crucial for predicting how elements interact, form compounds, and behave chemically.
At the top of the table, you’ll find elements like hydrogen, which constitutes about 90% of atoms. As you move down, you’ll encounter elements with higher atomic numbers, such as Oganesson. The atomic number indicates the number of protons in an element’s nucleus, with electrons orbiting around this nucleus.
Layout Specific to A-level
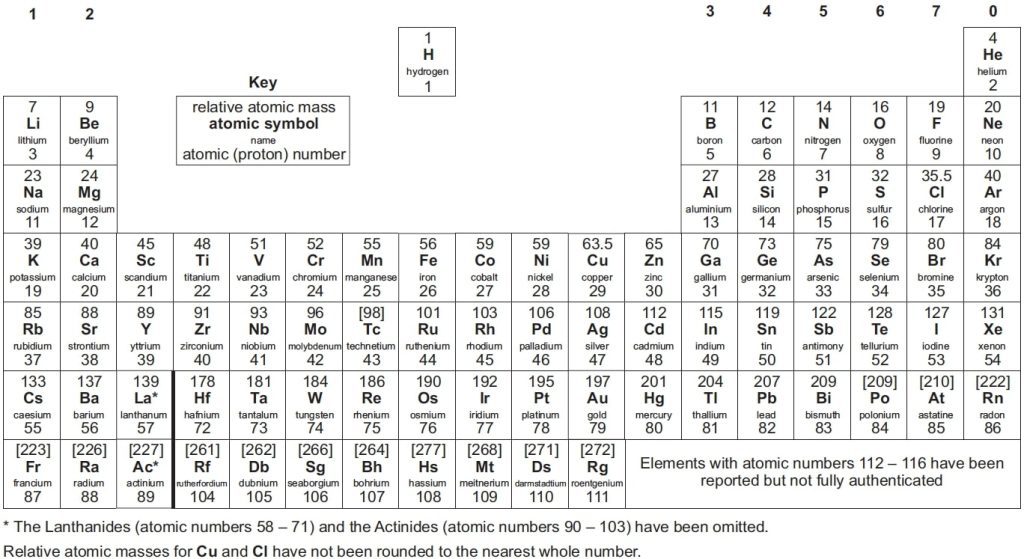
The layout of the A-level periodic table mirrors that of other periodic tables, including those used at the GCSE. The periodic table is divided into vertical columns known as groups and horizontal rows known as periods.
What are Groups in A-Level Chemistry?
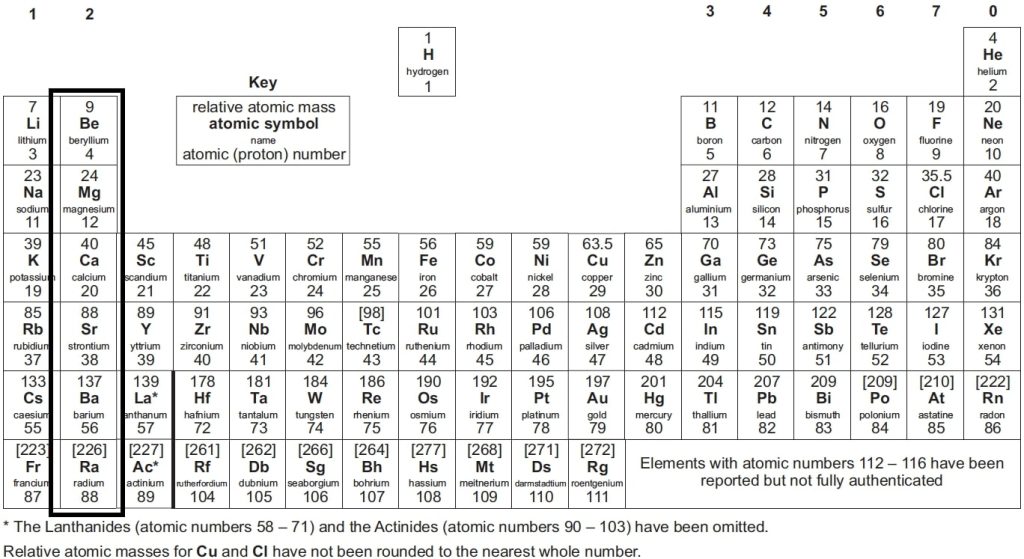
Elements in the same group share similar properties. For instance, Group 1 elements, known as alkali metals (e.g., lithium, sodium, potassium), are highly reactive. This shared reactivity is due to each element having the same number of electrons in its outermost shell.
Key Points:
- Elements in the same group exhibit similar chemical properties.
- Group members have the same number of outer shell electrons.
What are Periods in A-level Chemistry?
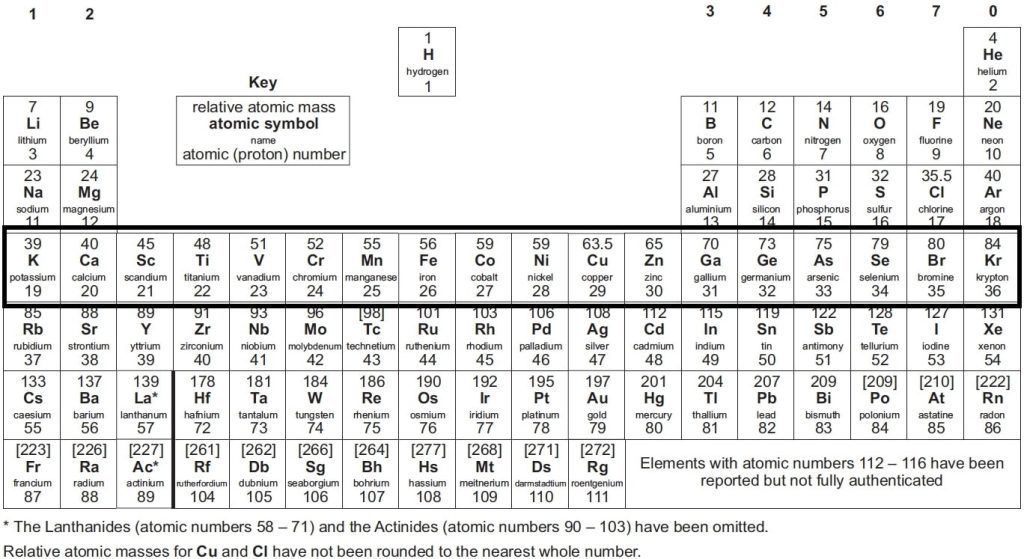
Elements are also organised into periods, which are horizontal rows on the table. Each period number signifies the number of atomic shells an element possesses. For example, lithium, located in period 2, has two electron shells.
Key Points:
- Periods indicate the number of electron shells.
- Elements in the same period have progressively increasing atomic numbers and electron shells.
Do You Need to Know the A-Level Periodic Table by Heart?
While memorising the entire periodic table is unnecessary, understanding how to read and interpret it is essential for A-Level chemistry. Knowing the characteristics of elements in specific groups or periods and the general layout will aid significantly in exams.
During A-Level chemistry exams, students are provided with a copy of the periodic table, so there’s no need to memorise it by heart. Familiarity with the table’s layout and the ability to quickly locate information is crucial.
Fun Facts About Chemistry
Chemistry is full of intriguing facts that can make learning more enjoyable:
- Mpemba Effect – Hot water can freeze faster than cold water.
- Water and Salt – Adding salt to a full glass of water makes the water level drop.
- Mixing Volumes – Mixing equal volumes of alcohol and water results in a total volume less than the sum of the individual volumes.
- Letter J – The periodic table does not include the letter J.
- Capsaicin – Birds are immune to the heat from hot peppers, unlike humans.
- Water Toxicity – Drinking excessive amounts of water can be fatal.
- Glass – Technically, glass is a liquid that flows very slowly.
- DNA – DNA acts as a flame retardant.
- Temperature Perception – Water feels colder than air at the same temperature.
- Molecular Structures – A car tyre is essentially one large molecule.
Conclusion
A-Level Chemistry is challenging but rewarding, with the periodic table at its core. Understanding this crucial tool not only aids in exams but also lays the groundwork for future scientific endeavours. With the help of a dedicated tutor, mastering the periodic table and other chemistry concepts can become a more manageable and enjoyable journey. Who knows, your exploration of the periodic table could inspire you to contribute to the discovery of the next element!
If you are searching for A-Level Chemistry tutors, consider using Edumentros. This online tutoring platform connects students and parents with tutors from top UK universities, ensuring high-quality educational support tailored to individual needs.