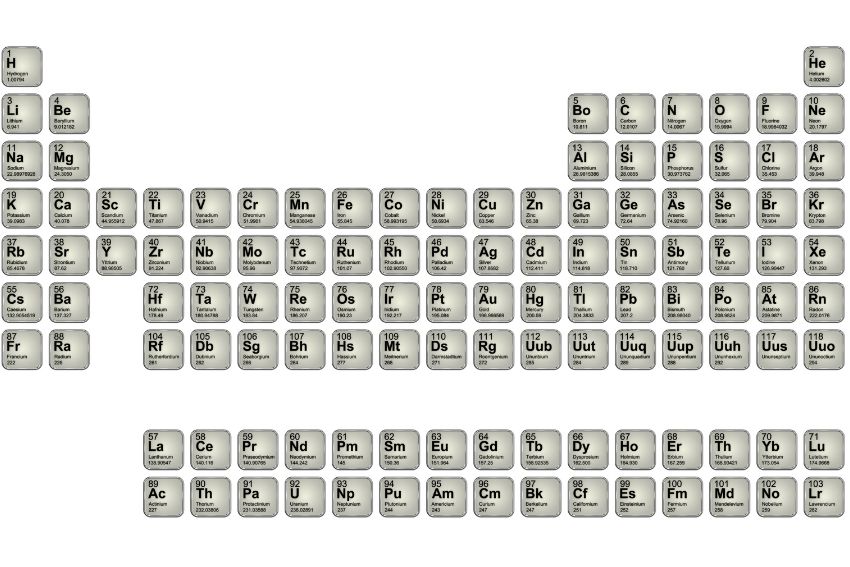
The periodic table KS3 is a key part of chemistry. It helps students understand the elements that make up everything around us. More than just a chart, it shows how elements behave and interact with each other. The periodic table was first arranged by Dmitri Mendeleev in 1869, who organised the elements based on their atomic masses and discovered that elements with similar properties appeared at regular intervals. By learning the periodic table, students can discover patterns and connections that make chemistry easier to understand.
Mastering the periodic table also prepares KS3 students for advanced topics like reactivity, bonding, and material classification. It not only improves exam performance but also sparks curiosity about the world around them.
In this blog, we’ve summed up everything you need to know about the periodic table at KS3. Let’s dive in and make learning easy and enjoyable!
What is the Structure of the Periodic Table KS3?
What does the periodic table show? the periodic table organises elements into rows (called periods) and columns (called groups), helping us understand their relationships. Let’s break it down:
Periods (Rows)
The periodic table has 7 periods. As you move across a period from left to right, the atomic number of elements increases. This means that elements become less metallic and more non-metallic as you move across the table.
For example, in Period 1, the first element is hydrogen (H), and the last element is neon (Ne). Hydrogen is a gas and a non-metal, while neon is a noble gas, also a non-metal, but with very different properties.
Groups (Columns)
The periodic table has 18 groups. Elements in the same group share similar chemical properties because they have the same number of electrons in their outer shell.
For example, Group 1 contains the alkali metals, like lithium (Li) and sodium (Na). These metals are highly reactive and share similar properties, like reacting with water.
Metals, Non-Metals, and Metalloids
The periodic table is also divided into metals, non-metals, and metalloids (elements that have properties of both metals and non-metals).
Metals are mostly on the left and centre, while non-metals are on the right side of the table. Metalloids, such as silicon (Si), sit between metals and non-metals.
The Transition Metals
The transition metals are found in the middle of the table, between groups 2 and 3. These elements have special properties, such as being good conductors of heat and electricity.
Understanding the structure of the periodic table helps KS3 students predict the properties of elements. This makes learning chemistry easier and more logical. It also shows how elements are connected and why some behave similarly.
Key Groups in the Periodic Table KS3
Now that we’ve looked at the structure of the periodic table. let’s take a look at three important groups that every KS3 student should know:
| Group | Elements | Properties | Example |
| Group 1: Alkali Metals | Lithium, Sodium, Potassium | Highly reactive, especially with water. Reactivity increases as you move down the group. | Sodium reacts with water to produce hydrogen gas and heat. |
| Group 7: Halogens | Fluorine, Chlorine, Bromine, Iodine | Non-metals that become less reactive as you go down the group. Atoms get larger, making it harder to attract electrons. | Fluorine is highly reactive, while iodine is less reactive. |
| Group 0: Noble Gases | Helium, Neon, Argon | Inert or unreactive due to full outer electron shells. Stable and unlikely to form bonds with other elements. | Helium is used in balloons because it is lighter than air and non-reactive. |
These key groups are part of the KS3 Chemistry curriculum. it will help you predict how different elements will behave in reactions, making chemistry more understandable and interesting.
How Do You Read the Periodic Table Easily?
Reading the periodic table might seem tricky at first, but once you understand its parts, it becomes much easier.

While memorising the entire periodic table KS3 isn’t necessary, understanding how to read and interpret it is an essential skill in chemistry. Knowing the characteristics of elements in specific groups, periods, and the overall layout will greatly help in exams.
At KS3, students don’t need to learn every detail of the periodic table by heart, but being familiar with its structure and how to find key information quickly is crucial. This understanding forms the foundation for more advanced chemistry topics, helping students approach the subject with confidence.
Tips for Learning the Periodic Table KS3
Learning the periodic table KS3 can feel challenging at first, but with the right techniques, it becomes much easier. Here are some helpful tips to make it more fun:
- Use Memory Techniques – Use flashcards to memorise element symbols, atomic numbers, and group names. Write the element on one side and key details on the other.
- Explore Study Tools and Apps – Take advantage of apps like Kahoot, Quizlet, or Periodic Table Quiz to make learning interactive. These platforms offer quizzes and games that test your knowledge in a fun way. Interactive website like BBC Bitesize provide engaging resources for KS3 students.
- Regular Practice with Periodic Table Charts – Keep a printed periodic table handy for reference and regular practice. Practice finding patterns, such as identifying trends in reactivity or grouping similar elements together.
- Try Quizzes and Mock Tests – Quizzes and mock tests help you apply what you’ve learned and pinpoint areas for improvement. Many online platforms offer free quizzes tailored to KS3 topics.
Using these tips, KS3 students can build a strong understanding of the periodic table. The more interactive and consistent the practice, the easier it becomes to master this essential tool in chemistry.
Fun Facts About the Periodic Table KS3
- Hydrogen is the lightest and most abundant element, making up 75% of the universe’s mass.
- Gold has been valued for thousands of years, used in ancient jewellery and coins.
- Dmitri Mendeleev named it the “Periodic Table” because of repeating patterns in elements.
- Helium changes how sound travels in your vocal cords, giving you a squeaky voice when inhaled.
- Mercury is the only metal liquid at room temperature, used in thermometers.
- Elements are named after places (Californium) or scientists (Curium).
- Oganesson, a “superheavy” element, is one of the rarest, created only in labs.
Conclusion
The periodic table KS3 is an important part of learning chemistry. It helps students understand elements and how they behave. Mastering the periodic table not only improves exam performance but also prepares students for more advanced science topics. Its patterns and connections make chemistry more interesting and turn it into an exciting way to explore the world around us.
To get the most out of your learning, try using interactive tools, practice quizzes, and fun periodic table activities. These can make studying more engaging and help you retain information better. And if you or your child needs extra support, consider exploring online tutoring in KS3 Chemistry. A tutor can provide personalised guidance and make tricky topics easier to understand. Good Luck!
FAQ’s
What is the smallest element?
Hydrogen (H) has the smallest atomic mass, made of protons and electrons. Atomic radius decreases across a period and increases down a group.
Do you get a periodic table in Chemistry KS3?
Yes, KS3 students are usually provided with a periodic table during their chemistry lessons and exams. While they’re not expected to memorise the entire table, they should understand how to read it, including recognising groups, periods, and element properties. This knowledge helps them answer questions about patterns, reactivity, and element behaviours.
What are core subject in KS3?
The core subjects at this stage include : Science, English, Maths.
What are the periodic trends in KS3 chemistry?
Reactivity:
- Group 1: Increases down the group (easier to lose electrons).
- Group 7: Decreases down the group (harder to gain electrons).
Atomic Size:
- Across a Period: Decreases (stronger pull from the nucleus).
- Down a Group: Increases (more electron shells).
Metallic and Non-Metallic Properties:
- Across a Period: Metallic decreases, non-metallic increases.
- Down a Group: Metallic properties become stronger.
Melting/Boiling Points:
Group 7: Increase down the group.
Group 1: Decrease down the group.